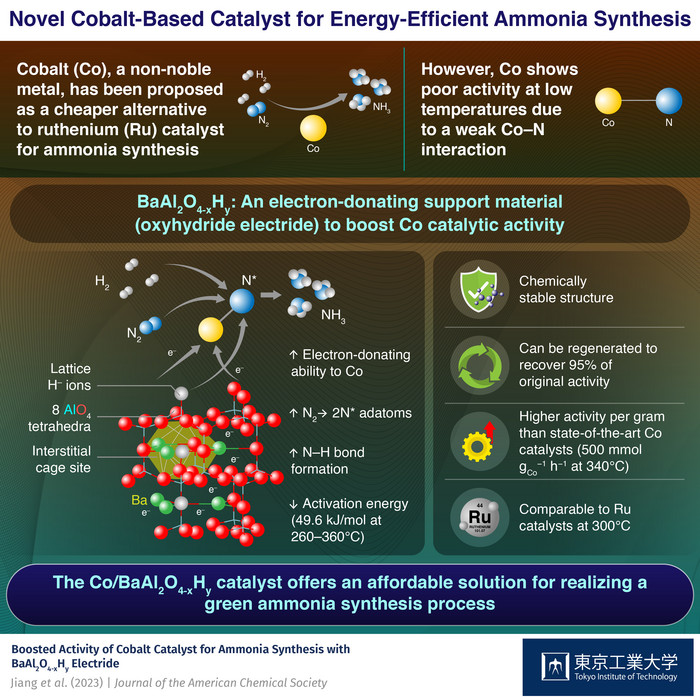
A New Era in Sustainable Chemical Production: Cobalt-Based Catalysts
The world relies heavily on chemical reactions for various industries, from pharmaceuticals to food production. Hydrogenation, a fundamental chemical reaction involving hydrogen gas and another compound, is essential in these industries. Traditionally, noble metals like palladium and rhodium are used as catalysts in these reactions. However, their scarcity, high cost, and environmental concerns associated with their mining make them unsustainable options.
Cobalt: A Promising Alternative to Noble Metals
In recent years, cobalt has emerged as a promising alternative to noble metal catalysts for hydrogenation. Cobalt nanoparticles (Co NPs) offer a more sustainable and cost-effective approach, capable of catalyzing hydrogenation reactions efficiently at lower temperatures and pressures compared to noble metal catalysts.
Unveiling the Potential of Cobalt: Crystal Phase Matters
The crystal phase of Co NPs plays a crucial role in their catalytic performance. However, studying this aspect has been challenging due to the lack of methods to produce Co NPs with a specific crystal phase of similar size.
Breakthrough in Cobalt Nanoparticle Synthesis: A Hydrosilane-Assisted Method
Researchers at Tokyo Institute of Technology and Osaka Metropolitan University have developed a groundbreaking method to synthesize Co NPs with controlled crystal phases, paving the way for tailored catalytic performance. Their findings, published in the Journal of the American Chemical Society, shed light on the potential of cobalt in sustainable chemical production.
Hydrosilane: A Key Enabler for Controlled Crystal Growth
The research team, led by Professor Michikazu Hara, utilized a technique called the hydrosilane-assisted method. This method leverages the unique properties of hydrosilanes, which act as both reducing agents for Co cations and ligands on the metal complexes, effectively controlling the growth of metal particles.
Two Distinct Crystal Phases: Unveiling the Differences in Catalytic Performance
Through careful experimentation, the researchers successfully synthesized two types of Co NPs: face-centered cubic (fcc) Co NPs and hexagonal close-packed (hcp) Co NPs. The catalytic performance of these two types of Co NPs was then compared in a series of hydrogenation experiments.
Hexagonal Close-Packed Cobalt Nanoparticles: A Superior Catalyst
The results were remarkable. The hcp-Co NPs outperformed their fcc-Co counterparts in the hydrogenation of benzonitrile into benzyl amine. Hcp-Co NPs achieved a significantly higher selectivity, resulting in a 97% yield compared to fcc-Co NPs’ 80%. Moreover, the hydrogenation reaction using hcp-Co NPs required only half the pressure compared to fcc-Co NPs, leading to a more energy-efficient process.
A New Horizon in Sustainable Chemistry
The groundbreaking findings of this research highlight the immense potential of cobalt-based catalysts for sustainable chemical production. The proposed catalytic system, utilizing abundant cobalt, is reusable and does not require harmful gases, making it a promising alternative to conventional methods. This development could significantly reduce the environmental impact and cost of producing various industrial commodities, drugs, and even food products, leading to a more sustainable future for humanity.
The Future of Cobalt-Based Catalysis: A Bright Outlook
This research marks a significant step forward in the pursuit of sustainable chemical production. The development of energy-efficient, reusable, and versatile catalytic systems based on abundant cobalt opens doors to a future where chemical industries operate more sustainably and responsibly. As scientists continue to explore the potential of cobalt-based catalysts, we can expect even more innovative solutions to emerge, contributing to a cleaner and more sustainable future for all.